1.0
Scope and Purpose
This
document outlines the general approach undertaken to identify, assess and
evaluate the chemical reaction hazards associated with chemical processes. It
should be noted that this document is not intended to be a standard operating
procedure, but an outline of the generalized working principles and
documentation of the rationale used.
This guideline is directed to
those personnel involved in research & development, process hazards
analysis (Process safety lab), kilo lab and commercial plant operations. It is
also useful to those involved in process hazard analysis and process safety
management.
2.0
Objective
·
increase
awareness of potential chemical reaction hazards associated with chemical
manufacture in batch and semi-batch processes
·
help in the
assessment of risks from chemical reactions, and advise on how to prevent and
control these risks
·
provide a
systematic approach for the design, operation and control of chemical reactions
in batch and semi-batch processes
·
advise on
safe management procedures and appropriate precautions to prevent or reduce
injuries and damage caused to property or the environment associated with
chemical manufacture; and
·
advise on
maintenance, training and information needs to prevent and control chemical
reaction hazards
3.0
Definition of terms
Activation energy: the constant Ea
in the exponential part of the Arrhenius equation associated with the minimum
energy difference between the reactants and an activated complex (transition
state), which has a structure intermediate to those of the reactants and the
products, or with the minimum collision energy between molecules that is
required to enable a reaction to take place; it is a constant that defines the
effect of temperature on reaction rate.
Adiabatic: a system condition in which no heat is
exchanged between the system and its surroundings; in practice, near adiabatic
conditions are reached through good insulation.
Adiabatic induction time: the delay time
to an event (spontaneous ignition, explosion, etc.) under adiabatic conditions
starting at operating conditions.
Adiabatic temperature rise: maximum
temperature increase (readily calculated, that can be achieved) which would occur
only when the substance or reaction mixture decomposes completely under
adiabatic conditions.
Apparent activation energy: the constant Ea
that defines the effect of temperature on the global reaction rate.
Autocatalytic reaction: reaction in
which the rate is increased by the presence of one or more of its intermediates
and/or products.
Batch reactor: reactor in which all reactants
and solvents are introduced prior to setting the operating conditions (e.g.,
temperature and pressure).
Decomposition energy: the maximum
amount of energy which can be released upon decomposition.
Decomposition temperature: temperature at
which decomposition of a substance occurs in a designated system; it depends
not only on the identity of the substance but also on the rate of heat gain or
loss in the system.
Deflagration: a release of energy caused by a rapid
chemical reaction in which the reaction front propagates by thermal energy
transfer at subsonic speed
Detonation: a release of energy caused by an extremely
rapid chemical reaction of a substance in which the reaction front propagates
by a shock wave at supersonic speed.
Endothermic reaction: a reaction is
endothermic if energy is absorbed; the enthalpy change for an endothermic
reaction is a positive value.
Enthalpy of reaction: the net
difference in the enthalpies of formation of all of the products and the
enthalpies of all of the reactants; heat is released if the net difference is
negative.
Exothermic reaction: a reaction is
exothermic if energy is released; the enthalpy change for an exothermic
reaction is a negative value.
Hazard: a chemical or physical condition that
has the potential for causing harm or damage to people, property, or the
environment.
Isothermal: a system condition in which the
temperature remains constant; this implies that heat internally generated or
absorbed is quickly compensated for by sufficient heat exchange with the
surroundings of the system.
Onset temperature: temperature at which a
detectable temperature increase is first observed due to a chemical reaction;
it depends entirely on the detection sensitivity of the specific instrument
involved; scale-up of onset temperatures and application of rules-of-thumb
concerning onset temperatures are subject to many errors.
Phi-factor: a correction factor which is based on
the ratio of the total heat capacity of a vessel and contents to the heat
capacity of the contents; the Phi-factor approaches one for large vessels.
Quenching: Abruptly stopping a reaction by severe
cooling or by catalyst inactivation in a very short time period; used to stop
continuing reactions in a process thus preventing further decomposition or
runaway.
Rate of reaction: technically, the rate at which
conversion of the reactants takes place; the rate of reaction is a function of
the concentrations and the reaction rate constant; in practical terms, it is an
ambiguous expression that can describe the rate of disappearance of reactants,
the rate of production of products, the rate of change of concentration of a
component, or the rate of change of mass of a component.
Runaway: a thermally unstable reaction system
which shows an accelerating increase of temperature and reaction rate which may
result in an explosion.
Time to maximum reaction rate: the measured time
to the maximum reaction rate during a runaway or rapid decomposition; the
specific result is highly contingent on the test method used.
Venting: an emergency flow of vessel contents
out of the vessel thus reducing the pressure and avoiding destruction of the
unit from over-pressuring; the vent flow can be single or multiphase, each of
which results in different flow and pressure characteristics.
1.0
Introduction
Any chemical process
will involve chemicals and its interactions and equipment in which it is processed.
This interaction of chemical within equipment possess some degree of hazard
which needs to be understood to control or eliminate the risk. Hence it is
important to understand what the parameters which determines safe chemical
process are and what are chemical reaction hazards.
4.1
Parameters
that determines the design of the safe chemical processes
Three parameters that
is chemicals (its intrinsic properties), Reactions rates (Kinetics) and
Hardware (equipment) determines the design of safe chemical process as depicted
below.
Fig 1: Parameters that determine safe chemical processes
· Potential energy of chemicals involved: Design of a safe process requires
an understanding of the inherent energy (exothermic release/endothermic
absorption) during chemical reactions. This information can come from the
literature, from thermochemical calculations, or from proper use of testing
equipment and procedures. The potential pressure that may be developed in the
process is also a very important design consideration.
· Rates of the reaction/ decompositions: this depends upon
temperature, pressure and concentration. Rates of reaction during the normal/
abnormal process conditions must be determined to design safer processes.
· Plant process equipment and
design:Any
heat that is generated by the reaction must be removed adequately, and any gas
production must be managed. The effects and requirements of scale-up (that is,
the relation between bench-scale and plant equipment) must be considered.
4.2
Chemical
Reaction Hazards
· Chemical Reaction Hazards are the hazards that result from uncontrolled chemical reactions.
· An uncontrolled chemical reaction can be
defined as one in which the heat generated by the reaction is greater than the
heat which can be removed to the surroundings (plant heat transfer systems)
This results in the temperature of the reaction mixture increasing, which results in an increase in the rate of reaction. This in turn leads
to a further increase in the rate of heat generation. When the
temperature reaches the boiling point (and/or) decomposition temperature,
pressure generation rate can exceed the venting capacities of the plant system
resulting in an explosion.
· Runaway
reactions can therefore start slowly but accelerate,
until finally it can result in an explosion.
Chemical Reaction Hazards are the result of 3 main parameters:
Ø The thermal instability of starting/raw materials,
reaction mixtures and products.
Ø Rapid exothermic reactions that raise the reaction
temperature to decomposition or violent boiling.
Ø Rapid gas evolution.
Fig 2: Overview of pathway of
Chemical Reaction Hazards
Note: It is the
pressure that results in a chemical reaction hazard incident and not the heat.
However, the uncontrolled heat leads to pressure generation by the above
scenarios.

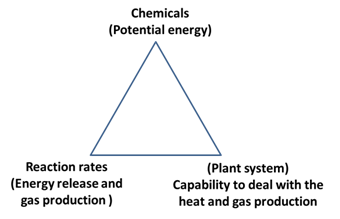


hi
ReplyDelete